Over the past two decades great improvements in therapy of chronic inflammatory diseases have been made. The rise of TNF biologics like infliximab has been a great step forward to ameliorating disease course and keep inflammations at remission levels for prolonged periods of time. Therapeutic Drug Monitoring (TDM) of biotherapies is a well recognized tool allowing a better usage of biotherapies by making evidence-based treatment decisions.
However, all the benefits of TDM for infliximab are not available on a true point-of-care platform. Current limitations in testing force dose adjustments to come weeks after trough level testing.
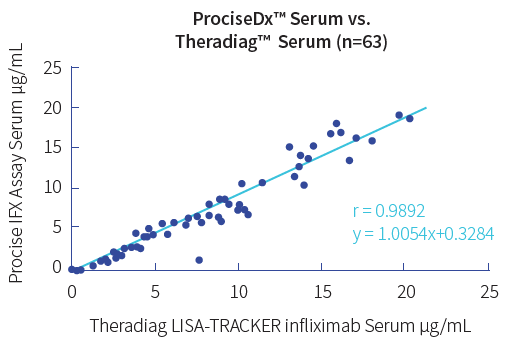
Procise IFX allows immediate decisions on drug dose adjustments at the time of infusion in only 5 minutes with finger prick blood by virtually any level laboratory technician.
Product Information
| Product Information | |
|---|---|
| Sample Types | Finger prick whole blood, venous blood, or serum |
| Reportable Range | 1.7μg/mL - 77.2μg/mL |
| Time to Results | Less than 5 minutes |
| Stability | 2 years at room temperature |
| Validated for | Infliximab (Remicade®, Remsima®, Inflectra®, Flixabi® and Renflexis®) |
Calibrated against WHO standard.
Performance
Precision
| Level | Avg[IFX]μg/mL | %CV |
|---|---|---|
| Low QC | 3.1 | 4.8% |
| High QC | 20.3 | 2.8% |
| Low Serum | 4.4 | 3.1% |
| Med Serum | 9.0 | 3.3% |
| High Serum | 36.5 | >2.8% |
Ordering Information
| Item | Cat # | Description | #/Kit |
|---|---|---|---|
| IFX Assay | 4828 | Kit for conducting assays | 20 |
| In addition to 20 tests, the kit includes: | |||
| 4403 | Buffer bulbs | 20 | |
| 4866 | IFX controls | 2 hi and low | |
| 4552 | Whole blood pipettes | 20 |
“With ProciseDx, we will be able to measure levels of infliximab and adalimumab in five minutes from fingerstick blood. This information during the patient visit will be incorporated into our immediate treatment decisions.”

Contact Us
To learn more about our assays or to speak with our team, please contact us here or email info@procisediagnostics.com.





![[PRO] 501 - ProciseDx Additional Brandmarks R2.00_IFX.png](https://images.squarespace-cdn.com/content/v1/5e1a8289c429c302ddea1459/1596491363091-X0TH8YMIP1UP4GWNJN1B/%5BPRO%5D+501+-+ProciseDx+Additional+Brandmarks+R2.00_IFX.png)